On November 15, the research group led by Prof. Yuan Weiming published an online paper entitled “From Esters to Ketones via a Photoredox-Assisted Reductive Acyl Cross-Coupling (PARAC) Strategy” on Angewandte Chemie International Edition.
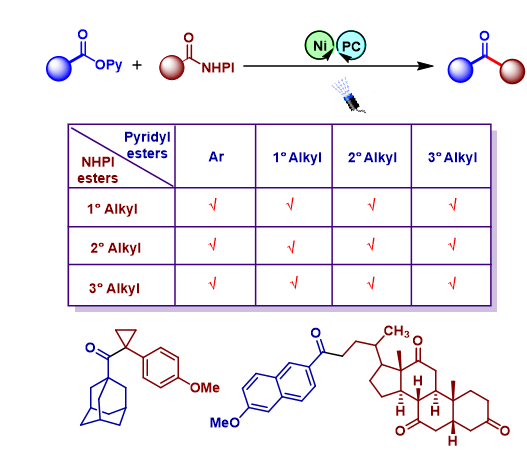
A nickel/photoredox dual-catalyzed cross-electrophile coupling of two different carboxylic acid esters towards the synthesis of ketones via a photoredox-assisted reductive acyl cross-coupling (PARAC) strategy is developed. A variety of aryl, 1 o , 2 o , 3 o -alkyl 2pyridyl esters can act as acyl electrophiles while N (acyloxy)phthalimides (NHPI esters) act as 1 o , 2 o , 3 o -radical precursors. Our PARAC strategy provides an alternative and reliable way to synthesize various sterically congested 3 o -3 o , 3 o -2 o , and aryl3 o ketones under mild and highly unified conditions, which have been otherwise difficult to access. The combined experimental and computational studies identified a Ni(0)/Ni(I)/Ni(III) pathway for ketone formation.
Huazhong University of Science and Technology is the first unit of this paper. Prof. Yuan Weiming and Prof. Qi Xiaotian of Wuhan University are the co-corresponding authors of the paper (theoretical calculation part). The first authors of the paper are PhD students Xi Xiaoxiang from the School of Chemistry and Chemical Engineering of Huazhong University of Science and Technology and Luo Yixin from Wuhan University (theoretical calculation part). This research work was funded by the Startup Fund of Huazhong University of Science and Technology.
Link to this paper: https://doi.org/10.1002/anie.202114117